Low battery
Battery level is below 20%. Connect charger soon.
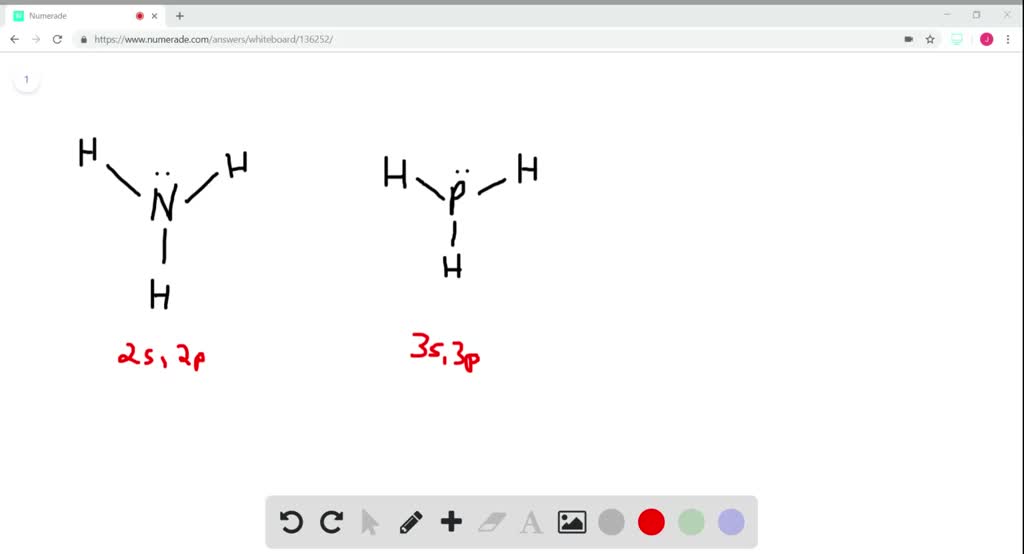
Because the ammonia molecule has four electron domains (the three electron clouds around ea. · in this video we look at the electron geometry for ammonia (nh3). Ammonia is soluble in … The ideal geometry for 4 electron domains is tetrahedral, but the lone pair compresses the angles between the hydrogen atoms. The three hydrogen … · in this article, we will discuss ammonia (nh3) lewis structure, molecular geometry or shape, electron geometry, bond angle, hybridization, formal charge, etc. As to accomplish this, the 4 electron pairs (or electronic domains) of nitrogen (the lone pair plus the three binding pairs) get the conformation of a tetrahedron. The resulting molecular shape is trigonal … This knowledge … Understand its trigonal pyramidal shape, bond angles, and lone pair effects, driven by vsepr … · even though the electron pair geometry is tetrahedral, the actual shape (molecular geometry) is determined only by the positions of atoms, not lone pairs. Ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair). · in the case of nh3, its electron geometry is tetrahedral, which, combined with the presence of a lone pair, results in a trigonal pyramidal molecular geometry. However, its molecular geometry is trigonal pyramidal because the bond angles are 107 degrees as the hydrogen atoms are repelled by the … Thus, the electron geometry of nh3 is tetrahedral. · by following these tips, you can accurately determine the electron geometry of ammonia (nh3) and understand the underlying principles that dictate its shape. · explore the molecular geometry of ammonia (nh3), a crucial concept in chemistry. The presence of the lone pair affects the shape. These are arranged in a tetrahedral shape. In this video we look at the electron geometry for ammonia (nh3).